momentum distribution particle in a box Particle in a Box Outline - Review: Schrödinger Equation - Particle in a 1-D Box. Eigenenergies . probability distribution of finding the particle. Schrodinger: A Wave Equation for Electrons. . - the probability of finding a particle with a particular momentum . The Wavefunction PHYSICALLY MEANINGFUL STATES MUST HAVE THE FOLLOWING . The yellow box junction markings are to try and prevent people not to chance going onto the level crossing if they cannot exit even when the barriers are up and the lights are .
0 · what is the momentum of a particle
1 · quantum particle in a box solutions
2 · quantum particle in a box explained
3 · quantum particle bound to a box
4 · particle in a box no momentum
5 · momentum of particle in box
6 · how to find momentum in a box
7 · how to calculate momentum of particle
Yankee Sheet Metal offers HVAC duct manufacturing services across .
What is the average momentum of a particle in the box? We start with Equation \(\ref{expect}\) and use the momentum operator \[\hat{p}_{x}= .
queen sized steel box spring
Explain why the energy of a quantum particle in a box is quantized; Describe the physical meaning of stationary solutions to Schrӧdinger’s equation and the connection of these solutions with time-dependent quantum states; Explain .In this paper, we focus on the momentum distributions for systems in energy eigenstates of the particle-in-a-box Hamiltonian, a widely used quantum-mechanical model (1-9). We obtain . The momentum operator in one dimensional quantum mechanics is: $$\hat p_x=\frac{\hbar}{i}\frac{d}{dx} $$ and we can imagine creating an eigenvalue-eigenfunction system $$\hat p_x\psi = p_x\psi.$$ As a student of ODE, I see here a Sturm-Liouville problem if we let $\lambda=-i\hbar p_x$ then we can say $\psi'+\lambda\psi=0$.If we then imposed .
what is the momentum of a particle
Particle in a Box Outline - Review: Schrödinger Equation - Particle in a 1-D Box. Eigenenergies . probability distribution of finding the particle. Schrodinger: A Wave Equation for Electrons. . - the probability of finding a particle with a particular momentum . The Wavefunction PHYSICALLY MEANINGFUL STATES MUST HAVE THE FOLLOWING .Show that the particle-in-a-box wavefunctions are not eigenfunctions of the momentum operator (Equation \(\ref{3.2.3a}\)). Answer The easiest way to address this question is asking if the PIB wavefunction also satisfies the .
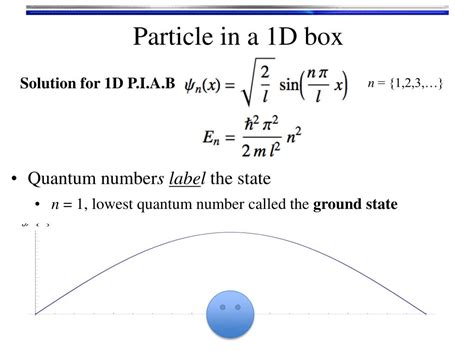
Classical vs Quantum particle in a box#. Particle in a box is a toy model of electron (or atom, molecule, small quantum object) trapped in some region of space \([0,L]\).. The positional information of a quantum “particle” is described by a quantum wave function \(\psi(x)\) which is obtained by solving Schrödinger equation with boundary conditions . The probability distribution for a particle in a box at the \(n=1\) and \(n=2\) energy levels looks like this: Notice that the number of nodes (places where the particle has zero probability of being located) increases with increasing energy n. Also note that as the energy of the particle becomes greater, the quantum mechanical model breaks . The momentum distribution of a quantum mechanical system is a crucial concept in understanding the behavior of particles at the microscopic level. . Let’s consider a few examples to illustrate the process of finding the momentum distribution. Example 1: Particle in a Box. For a particle confined in a one-dimensional box of length L, the . The energy eigenfunction in the one-dimensional box looks like $$\phi_n(x)=\sqrt{\frac{2}{L}}\sin\left( \frac{n\pi x}{L}\right)$$ which is a square integrable as in should be so that $\phi_n(x)\in l_2$ that suggest that the $\tilde{\phi}_n(p)$ must be square integrable too which is fourier transform of $\phi_n(x)$. OP's suggestion that $\tilde{\phi}_n(p) .
quantum particle in a box solutions
quantum particle in a box explained
Consider a particle in a box with infinite barriers. By solving the Schrödinger we can find the probability of finding the particle at some points in the box. . $\begingroup$ Heisenberg uncertainty principle could be useful to get an approximation for momentum distribution? $\endgroup$ – SebiSebi. Commented Jun 14, 2015 at 10:53 .
The index n is called the energy quantum number or principal quantum number.The state for is the first excited state, the state for is the second excited state, and so on. The first three quantum states (for of a particle in a box are shown in .. The wave functions in are sometimes referred to as the “states of definite energy.” Particles in these states are said to occupy energy levels .
The average initial speed of each particle is 0 ms^{-1}$. Estimate the timescale for the system to return close to its initial state so that each particle is within Question: Find the probability distribution for different values of the momentum of a particle in a potential box with infinite walls in the 1st energy state. Find the probability distribution for different values of the momentum of a particle in a potential box with infinite walls in the 1st energy state..1 cm$ of its initial location and with a momentum vector $\mathbf{p}$ satisfying $|\mathbf{p} − \mathbf{p}_{initial}| < 7.8 × 10^{-26} kg m s^{-1}$.
10 energy levels of the particle in a one‐dimensional box are displayed below. They show the probability that the particle will be found to have various momentum values in an . For example, for n = 10 the momentum distribution has principle maxima around +/‐ 30, suggesting a particle moving to the right and left with a specific momentum. . This operator can be derived from the momentum operator based on the relationship between momentum and kinetic energy that comes from classical physics. . which says that the square of the wavefunction must give a probability distribution as to where the particle can be measured to be. Since all measurements must place the particle in the box . According to Wikipedia, momentum for the 1D particle-in-a-box has a distribution: https: . Momentum of particle in a box explains it - QM is incompatible with de Broglie's matter wave theory apparently. Good to know. Also although energy states are quantized, this does not appear to be the case for kinetic energy. .The Schrödinger wave equation for a particle in a box. The particle in a box model lets us consider a simple version of the Schr ö dinger equation. Before we simplify, let's take another look at the full Hamiltonian for a particle-wave in .
It states that we cannot know both the position and momemtum of a quantum particle with complete certainty. We will show how this relationship can be derived from the results of the 1D particle in a box. Additionally, we will show how the particle in a box model can be applied to make predictions on real systems. 4.5.5.2. Learning Goals:#Consider the particle in a 1-d box, we know very well the solutions of it. . and when you go to measure the distribution with a physical instrument you introduce a resolution. . Particle in a box: simultaneously bounded momentum and .
Suppose we measure the momentum of a particle in a box and the wavefunction collapses to: $${| {p'} \rangle}.$$ We know that the particle cannot be found outside the box (infinite square well) . . and the probability distribution of momentum it implies is consistent with this result of measurement. This means the state after momentum . Concerning Momentum distribution of a particle in a box,-in case the world is x(- infinity, + infinity): b. continuous values whose probability is calculated from Fourier transform of wave function are observed.,-in case the world is x[0,L]: c. the momentum operator does not exist.
10 energy levels of the particle in a one‐dimensional box are displayed below. They show the probability that the particle will be found to have various momentum values in an . For example, for n = 10 the momentum distribution has principle maxima around +/‐ 30, suggesting a particle moving to the right and left with a specific momentum. .FOOTNOTE 1 The following animations show that as time goes on the probability distribution spreads over the whole box. FOOTNOTE 2 Observe that in the third animation, . In the absence of potentials, e.g. if there are no forces acting on the particle, the momentum is a conserved quantity. Therefore the uncertainty in the momentum is also constant.For both massive and massless particles in a box, the states of a particle are enumerated by a set of quantum numbers [n x, n y, n z].The magnitude of the momentum is given by = + +,, =,,, . where h is the Planck constant and L is the length of a side of the box. Each possible state of a particle can be thought of as a point on a 3-dimensional grid of positive integers.
Analysis of momentum distributions for systems in energy eigenstates of the particle-in-a-box Hamiltonian.
quantum particle bound to a box
Take a unit box, the energy eigenfunctions are $\sin(n\pi x)$ (ignoring normalization constant) inside the box and 0 outside. I have read that there is no momentum operator for a particle in a box,.First of all, I assume that you know that the probability (density) distribution is given by the squared amplitude of the wavefunction. In your example, you are given the wavefunction in the position basis, so it gives you the position probability density. If you want the momentum probability density, you have to change basis to get $\psi(p)$.. The standard way to change . A momentum eigenstate is a state in which a particle's momentum is precisely defined. This means that the particle's momentum has a specific value and cannot be measured to be any other value. 2. How does a particle in a box exhibit momentum eigenstates? In the case of a particle in a one-dimensional box, the particle is confined to a finite .Momentum variance in momentum space for particle in a box. Ask Question Asked 11 years, 1 month ago. Modified 8 years, 11 months ago. Viewed 4k times 2 $\begingroup$ My assignment asks me to compute the momentum space wavefunction of the nth energy eigenstate of the particle in a one-dimensional infinite square well, then "show that your result .
particle in a box no momentum
The HC rule says that "you must not enter a yellow box until your exit road or lane is clear. However, you may enter the box when you want to turn right, and are only preventing .
momentum distribution particle in a box|how to calculate momentum of particle